Hughes & Coleman Injury Lawyers is investigating on behalf of patients who suffer from the threat of pulmonary embolism and have received an Inferior Vena Cava filter or IVC to prevent pulmonary embolism.
Since the introduction of the IVC filter in 2005, the FDA has received roughly one thousand adverse reports involving these filters and reported that the IVC filters fragment and embolize in the body.
The FDA has urged physicians who advise their patients to use an IVC filter to consider the risk and benefits for individual patients instead of simply choosing the IVC for all patients who are ineligible for anticoagulation therapy, and to remove the device once the symptoms have diminished.
IVC filter complications are very serious and can be life threatening. Out of the 921 reported events to the FDA, there were:
- 328 device migrations
- 146 involved embolizations or detachment of the device components
- 70 IVC perforations
- 56 IVC filter fractures
Inferior Vena Cava Filters (IVC) filters are implanted into patients who have a history or risk of developing blood clots in the legs or pelvis. The devices were designed to catch fragments of blood clots that form in the legs or pelvis before they reach the heart, lungs, or brain and result in severe health complications.
IVC filters are under increased scrutiny due to high risks of failure. Defective IVC filters have shown complications of filter fracture, migration and perforation of vital organs. Malfunctions may result in death or serious injuries requiring corrective surgeries. At times, the devices can migrate to areas of the body that aren’t reachable by surgery.
IVC devices fail in some patients and migrates from the site of the implantation, leading to serious injuries. These injuries include:
- Perforations of the vena cava sometimes puncturing the aorta
- Migration of the device (metallic) to the heart (usually requiring open heart surgery)
- Fractures where the pieces embolize throughout the body, usually the heart.
Products where these problems have been reported include:
- Cook Celect IVC filters
- Cook Tulip IVC filters
- Cook Platinum IVC filters
- Bard Recovery Filter System
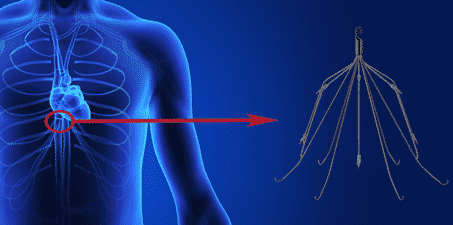
IVC stands for inferior vena cava, a large vein with the primary function of transporting de-oxygenated blood to the heart from the lower body. Also known as the posterior vena cava, this vein is connected to the major veins in the legs, and runs behind the abdominal cavity, along the spine. It connects to the lower right atrium of the heart, where the de-oxygenated blood is delivered through to the lungs to become oxygenated.
An IVC filter is a device that is implanted in the inferior vena cava to trap blood clots so they cannot reach the heart or lungs. It replaced the older technique of clipping, ligation or plication of the vein, which was common until the 1970s.
The current IVC filter design is based on one that was introduced in 1973. Constructed from stainless steel, its shape may resemble a partially closed umbrella frame with multiple, spidery prongs. Current models may also be constructed from titanium and be shaped like a cage or fence. The prongs or grates of the device serve to catch blood clots traveling by, preventing them from moving through the vein to the heart and lungs.
The majority of IVC filters are only intended for permanent implantation. However, in recent years, manufacturers have been creating IVC filters for temporary placement. These are known as “retrievable” and can be removed once the threat of blood clots is no longer considered a risk.
An IVC filter is used for patients who have issues with blood clots, most commonly for those diagnosed with deep venous thrombosis. Deep venous thrombosis (DVT) is a serious health condition characterized by the formation of blood clots in the lower leg, thigh or pelvic area. These blood clots can break up into smaller pieces and travel up through the inferior vena cava. Once they reach the heart, the blood clots can block one of the pulmonary arteries that deliver blood to the lungs, a condition known as pulmonary embolism (PE). DVT and PE commonly occur together, which is why they are collectively known as venous thromboembolism. Individuals at the highest risk are those who have sustained vein injury, slow blood flow, increased estrogen levels, chronic medical illnesses, or are obese. Genetics also plays a role.
- Current statistics compiled by the Centers for Disease Control and Prevention estimate that 90,000 people may be affected by DVT/PE every year.
- A study in the UK medical journal, Lancet, reports that pulmonary embolism (PE) occurs in as many as 40% of all proximal deep vein thrombosis (DVT) when left untreated.
- A study in the American Journal of Preventative Medicine reports that half of DVT/PE patients experience a reoccurrence within 10 years.
If you have been diagnosed with acute blood clotting issues which cannot be safely and effectively treated with anticoagulation medication, or have experienced treatment failure with this method, an IVC filter may be prescribed to you.
An IVC filter is placed within the vein using a catheter, which is inserted through the skin in the neck or groin. The catheter is positioned so that the IVC filter can access the vein. Once placed correctly inside the vein, the device is released. The filter expands and attaches itself to the vein’s walls.
Manufacturers design optionally retrievable filters to be permanent or temporary. They can be removed or left in place.
In May 2014, the FDA updated their initial communication regarding IVC filters. There have been several concerns expressed by the medical community regarding retrievable IVC filters and long-term complications. As a result, the FDA now recommends that retrievable IVC filters should be removed once they are no longer needed. This should be done as long as it is safe to do so given the health status of the patient. Based on a decision analysis published in a 2013 issue of the Journal of Vascular Surgery: Venous and Lymphatic Disorders, it is recommended that removal of the device takes place between 29 and 54 days after implantation.
Removal of the filter involves the use of a catheter. The catheter hooks on to the filter and closes it, so that it may be removed smoothly from the vein and body.
The FDA is continuing to compile additional clinical data on IVC filters that are available in the country for use today. Both permanent and retrievable models are being examined.
How can we help?
By calling us immediately, we will ask you a few questions and if you qualify, we will send you a packet in the mail for you to sign. We want to help you get the money you deserve and keep in mind that we have won millions for our clients over the years and we want to help you. Simply call us or fill out the form on our website. 800-800-4600
Cases likely to be referred.


